125,000+ SATISFIED CUSTOMERS
125,000+ SATISFIED CUSTOMERS
March 05, 2022
Our research shows only 1-in-500 probiotics on the market today are based on evidence they actually provide a health benefit.

However, the definition of a probiotic is a live microorganism that delivers a health benefit when consumed or applied to the body in a specific amount.
A probiotic is a live microorganism that delivers a health benefit when consumed or applied to the body in a specific amount.
A list of evidence-based probiotic brands can be viewed here.
"In the case of probiotics, the clinical evidence must be linked to specific formulations as defined by genus, species, alphanumeric designation or strain, number of live bacteria present, the blend of probiotic strains present and finally, non-active ingredients present."
Probiotics require evidence from double-blind, placebo-controlled trials that show their safety and efficacy by delivering a specific health related outcome.
To put it another way, the Board of Directors from the International Scientific Association for Probiotics and Prebiotics state "Clinical evidence and not microbiota outcomes drive the value of probiotics." In simple terms, clinical evidence and not a CFU count drive value of any probiotic.
The value of any probiotic is derived by the evidence of the probiotic strain(s) delivering a health benefit in a randomized controlled clinical trial.
What is a probiotic strain exactly? It’s the identifying numbers and/or letters after the probiotic species. It's most important to know that a species is not a strain.

A highly specific probiotic strain coupled with the same CFU count used in the randomized controlled trial is where evidence and value are derived.
Our probiotic strains designed for skin are backed by 6 human clinical trials
Translation: Our all-star probiotic blend works where you need it - your skin.
100% of Skinesa’s probiotic strains have been studied in human clinical trials. 1,2,3,4,5,6
We've carefully selected probiotic strains for skin backed by published science and deliver them to your door.
Are the probiotic strains you’re taking linked to published science? If not, they do not meet the criteria to be called a probiotic.
Evidence shows the ingredients in Skinesa® are to be effective for 92% of participants. 2
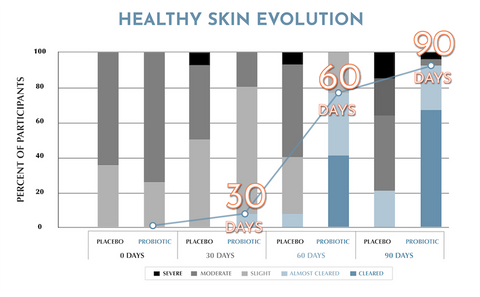
In fact, the probiotic strains in Skinesa® have been the subject of rigorous double-blind placebo controlled studies of over 100 patients in dermatology clinics. 2
We guarantee satisfaction within 90 days or your money back.
Skinesa® contains the one and only probiotic blend that has been published in the world's leading American dermatology journal. 1
With continuous daily use, the probiotic blends contained in Skinesa® have demonstrated efficacy in supporting clear, healthy skin in rigorous, double-blind, placebo-controlled clinical trials! 1,2
1. Navarro-López V, Ramírez-Boscá A, Ramón-Vidal D, et al. Effect of Oral Administration of a Mixture of Probiotic Strains on SCORAD Index and Use of Topical Steroids in Young Patients With Moderate Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2018;154(1):37–43. doi:10.1001/jamadermatol.2017.3647
2. Navarro-López, V., Martínez-Andrés, A., Ramírez-Boscá, A., Ruzafa-Costas, B., Núñez-Delegido, E., Carrión-Gutiérrez, M. A., Prieto-Merino, D., Codoñer-Cortés, F., Ramón-Vidal, D., Genovés-Martínez, S., Chenoll-Cuadros, E., Pérez-Orquín, J. M., Picó-Monllor, J. A., & Chumillas-Lidón, S. (2019). Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta dermato-venereologica, 99(12), 1078–1084. https://doi.org/10.2340/00015555-3305
3. Plaza-Díaz J, Fernández-Caballero JÁ, Chueca N, García F, Gómez-Llorente C, Sáez-Lara MJ, Fontana L, Gil Á. Pyrosequencing Analysis Reveals Changes in Intestinal Microbiota of Healthy Adults Who Received a Daily Dose of Immunomodulatory Probiotic Strains. Nutrients. 2015; 7(6):3999-4015. https://doi.org/10.3390/nu7063999
4. Olivares, M., Castillejo, G., Varea, V., & Sanz, Y. (2014). Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. British Journal of Nutrition, 112(1), 30-40. https://doi.org/10.1017/S0007114514000609
5. Pedret, A., Valls, R.M., Calderón-Pérez, L. et al. Effects of daily consumption of the probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes 43, 1863–1868 (2019). https://doi.org/10.1038/s41366-018-0220-0
6. Muñoz-Quezada, S., Bermúdez-Brito, M., Gómez-Llorente, C., Matencio, E., Bernal, M., Romero, F., & Gil, A. (2013). Lactobacillus rhamnosus CNCM I-4036 decrease inflammatory cytokine release in human intestinal epithelial cells induced by enterotoxigenic Escherichia coli. Proceedings of the Nutrition Society, 72(OCE1), E64. https://doi.org/10.1017/S0029665113000669
Comments will be approved before showing up.
November 29, 2023 8 Comments

These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease
